 | Info
Sheets |
| | | | | | | | | | | | | | | | | | | | | | | | |
 | Out-
side |
| | | | |
|
| | | | |
Result : Searchterm 'Liver MRI' found in 0 term [ ] and 3 definitions [ ] and 3 definitions [ ], (+ 19 Boolean[ ], (+ 19 Boolean[ ] results ] results
| 1 - 5 (of 22) nextResult Pages :  [1] [1]  [2 3 4 5] [2 3 4 5] |  | |  | Searchterm 'Liver MRI' was also found in the following services: | | | | |
|  |  |
| |
|
Liver imaging can be performed with sonography, computed tomography (CT) and magnetic resonance imaging ( MRI). Ultrasound is, caused by the easy access, still the first-line imaging method of choice; CT and MRI are applied whenever ultrasound imaging yields vague results. Indications are the characterization of metastases and primary liver tumors e.g., benign lesions such as focal nodular hyperplasia (FNH), adenoma, hemangioma and malignant lesions (cancer) such as hepatocellular carcinomas (HCC).
The decision, which medical imaging modality is more suitable, MRI or CT, is dependent on the different factors. CT is less costly and more widely available; modern multislice scanners provide high spatial resolution and short scan times but has the disadvantage of radiation exposure.
With the introduction of high performance MR systems and advanced sequences the image quality of MRI for the liver has gained substantially. Fast spin echo or single shot techniques, often combined with fat suppression, are the most common T2 weighted sequences used in liver MRI procedures.
Spoiled gradient echo sequences are used as ideal T1 weighted sequences for evaluating of the liver. The repetition time (TR) can be sufficiently long to acquire enough sections covering the entire liver in one pass, and to provide good signal to noise. The TE should be the shortest in phase echo time (TE), which provides strong T1 weighting, minimizes magnetic susceptibility effects, and permits acquisition within one breath hold to cover the whole liver. A flip angle of 80° provides good T1 weighting and less of power deposition and tissue saturation than a larger flip angle that would provide comparable T1 weighting.
Liver MRI is very dependent on the administration of contrast agents, especially when detection and characterization of focal lesions are the issues. Liver MRI combined with MRCP is useful to evaluate patients with hepatic and biliary disease.
Gadolinium chelates are typical non-specific extracellular agents diffusing rapidly to the extravascular space of tissues being cleared by glomerular filtration at the kidney. These characteristics are somewhat problematic when a large organ with a huge interstitial space like the liver is imaged. These agents provide a small temporal imaging window (seconds), after which they begin to diffuse to the interstitial space not only of healthy liver cells but also of lesions, reducing the contrast gradient necessary for easy lesion detection. Dynamic MRI with multiple phases after i.v. contrast media (Gd chelates), with arterial, portal and late phase images (similar to CT) provides additional information.
An additional advantage of MRI is the availability of liver-specific contrast agents (see also Hepatobiliary Contrast Agents). Gd-EOB-DTPA (gadoxetate disodium, Gadolinium ethoxybenzyl dimeglumine, EOVIST Injection, brand name in other countries is Primovist) is a gadolinium-based MRI contrast agent approved by the FDA for the detection and characterization of known or suspected focal liver lesions.
Gd-EOB-DTPA provides dynamic phases after intravenous injection, similarly to non-specific gadolinium chelates, and distributes into the hepatocytes and bile ducts during the hepatobiliary phase. It has up to 50% hepatobiliary excretion in the normal liver.
Since ferumoxides are not eliminated by the kidney, they possess long plasmatic half-lives, allowing circulation for several minutes in the vascular space. The uptake process is dependent on the total size of the particle being quicker for larger particles with a size of the range of 150 nm (called superparamagnetic iron oxide). The smaller ones, possessing a total particle size in the order of 30 nm, are called ultrasmall superparamagnetic iron oxide particles and they suffer a slower uptake by RES cells. Intracellular contrast agents used in liver MRI are primarily targeted to the normal liver parenchyma and not to pathological cells. Currently, iron oxide based MRI contrast agents are not marketed.
Beyond contrast enhanced MRI, the detection of fatty liver disease and iron overload has clinical significance due to the potential for evolution into cirrhosis and hepatocellular carcinoma. Imaging-based liver fat quantification (see also Dixon) provides noninvasively information about fat metabolism; chemical shift imaging or T2*-weighted imaging allow the quantification of hepatic iron concentration.
See also Abdominal Imaging, Primovistâ„¢, Liver Acquisition with Volume Acquisition (LAVA), T1W High Resolution Isotropic Volume Examination (THRIVE) and Bolus Injection.
For Ultrasound Imaging (USI) see Liver Sonography at Medical-Ultrasound-Imaging.com. | | | | | | | | | | | | | • Share the entry 'Liver Imaging':    | | | | | | | | | |  Further Reading: Further Reading: | | Basics:
|
|
News & More:
|  |
Utility and impact of magnetic resonance elastography in the clinical course and management of chronic liver disease
Saturday, 20 January 2024 by www.nature.com |  |  |
Even early forms of liver disease affect heart health, Cedars-Sinai study finds
Thursday, 8 December 2022 by www.eurekalert.org |  |  |
For monitoring purposes, AI-aided MRI does what liver biopsy does with less risk, lower cost
Wednesday, 28 September 2022 by radiologybusiness.com |  |  |
Perspectum: High Liver Fat (Hepatic Steatosis) Linked to Increased Risk of Hospitalization in COVID-19 Patients With Obesity
Monday, 29 March 2021 by www.businesswire.com |  |  |
EMA's final opinion confirms restrictions on use of linear gadolinium agents in body scans
Friday, 21 July 2017 by www.ema.europa.eu |  |  |
T2-Weighted Liver MRI Using the MultiVane Technique at 3T: Comparison with Conventional T2-Weighted MRI
Friday, 16 October 2015 by www.ncbi.nlm.nih.gov |  |  |
EORTC study aims to qualify ADC as predictive imaging biomarker in preoperative regimens
Monday, 4 January 2016 by www.eurekalert.org |  |  |
MRI effectively measures hemochromatosis iron burden
Saturday, 3 October 2015 by medicalxpress.com |  |  |
Total body iron balance: Liver MRI better than biopsy
Sunday, 15 March 2015 by www.eurekalert.org |
|
| |
|  | |  |  |  |
| |
|
MultiHance® is a paramagnetic contrast agent for use in diagnostic magnetic resonance imaging ( MRI) of the liver and central nervous system. MultiHance® is a small molecular weight chelate, which tightly binds the Gd atom. The substance is excreted partly by the kidneys, partly by the biliary system, which is especially unique.
MultiHance® is indicated, for the detection of focal liver lesions in patients with known or suspected primary liver cancer (e.g. hepatocellular carcinoma) or metastatic disease.
MultiHance® is also indicated in brain MRI and spine MRI where it improves the detection of lesions and provides diagnostic information additional to that obtained with unenhanced MRI.
Gd-BOPTA-enhanced MRA can provide superior vascular signal intensity and SNR, as compared with Gd-DTPA, due to its higher relaxivity, even at lower doses.
1 ml of solution MultiHance® contains: (0.5M) gadobenate dimeglumine 529 mg = gadobenic acid 334 mg + meglumine 195 mg. Viscosity at 37°C: 5.3 mPa
WARNING: NEPHROGENIC SYSTEMIC FIBROSIS
Gadolinium-based contrast agents increase the risk for nephrogenic systemic fibrosis (NSF) in patients with acute or chronic severe renal insufficiency (glomerular filtration rate less than 30 mL/min/1.73m 2), or acute renal insufficiency of any severity due to the hepato-renal syndrome or in the perioperative liver transplantation period. Drug Information and Specification T1, predominantly positive enhancement r1=9.7, r2=12.5, B0=0.5 T PHARMACOKINETIC Extracellular, hepatobiliary PREPARATION Solution for injection DEVELOPMENT STAGE For sale PRESENTATION Vials of 5, 10, 15 and 20 mL, 50 and 100 mL Multipacks (Pharmacy Bulk Package)
DO NOT RELY ON THE INFORMATION PROVIDED HERE, THEY ARE
NOT A SUBSTITUTE FOR THE ACCOMPANYING PACKAGE INSERT! Distribution Information TERRITORY TRADE NAME DEVELOPMENT
STAGE DISTRIBUTOR Australia MultiHance® for sale | |  | |
• View the DATABASE results for 'MultiHance®' (9).
| | |
• View the NEWS results for 'MultiHance®' (1).
| | | | |  Further Reading: Further Reading: | Basics:
|
|
News & More:
| |
| |
|  | |  |  |  |
| |
|
(SENSE) A MRI technique for relevant scan time reduction. The spatial information related to the coils of a receiver array are utilized for reducing conventional Fourier encoding. In principle, SENSE can be applied to any imaging sequence and k-space trajectories. However, it is particularly feasible for Cartesian sampling schemes. In 2D Fourier imaging with common Cartesian sampling of k-space sensitivity encoding by means of a receiver array enables to reduce the number of Fourier encoding steps.
SENSE reconstruction without artifacts relies on accurate knowledge of the individual coil sensitivities. For sensitivity assessment, low-resolution, fully Fourier-encoded reference images are required, obtained with each array element and with a body coil.
The major negative point of parallel imaging techniques is that they diminish SNR in proportion to the numbers of reduction factors.
R is the factor by which the number of k-space samples is reduced. In standard Fourier imaging reducing the sampling density results in the reduction of the FOV, causing aliasing. In fact, SENSE reconstruction in the Cartesian case is efficiently performed by first creating one such aliased image for each array element using discrete Fourier transformation (DFT).
The next step then is to create a full-FOV image from the set of intermediate images. To achieve this one must undo the signal superposition underlying the fold-over effect. That is, for each pixel in the reduced FOV the signal contributions from a number of positions in the full FOV need to be separated. These positions form a Cartesian grid corresponding to the size of the reduced FOV.
The advantages are especially true for contrast-enhanced MR imaging such as
dynamic liver MRI (liver imaging) ,
3 dimensional magnetic resonance angiography (3D MRA), and magnetic resonance cholangiopancreaticography ( MRCP).
The excellent scan speed of SENSE allows for acquisition of two separate sets of hepatic MR images within the time regarded as the hepatic arterial-phase (double arterial-phase technique) as well as that of multidetector CT.
SENSE can also increase the time efficiency of spatial signal encoding in 3D MRA. With SENSE, even ultrafast (sub second) 4D MRA can be realized.
For MRCP acquisition, high-resolution 3D MRCP images can be constantly provided by SENSE. This is because SENSE resolves the presence of the severe motion artifacts due to longer acquisition time. Longer acquisition time, which results in diminishing image quality, is the greatest problem for 3D MRCP imaging.
In addition, SENSE reduces the train of gradient echoes in combination with a faster k-space traversal per unit time, thereby dramatically improving the image quality of single shot echo planar imaging (i.e. T2 weighted, diffusion weighted imaging). | |  | |
• View the DATABASE results for 'Sensitivity Encoding' (12).
| | | | |  Further Reading: Further Reading: | News & More:
|
|
| |
|  |  | Searchterm 'Liver MRI' was also found in the following services: | | | | |
|  |  |
| |
|
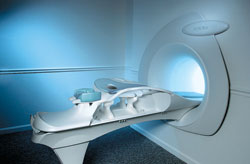
From Aurora Imaging Technology, Inc.;
The Aurora® 1.5T Dedicated Breast MRI System with Bilateral SpiralRODEO™ is the first and only FDA approved MRI device designed specifically for breast imaging. The Aurora System, which is already in clinical use at a growing number of leading breast care centers in the US, Europe, got in December 2006 also the approval from the State Food and Drug Administration of the People's Republic of China (SFDA).
'Some of the proprietary and distinguishing features of the Aurora System include: 1) an ellipsoid magnetic shim that provides coverage of both breasts, the chest wall and bilateral axillary lymph nodes; 2) a precision gradient coil with the high linearity required for high resolution spiral reconstruction;; 3) a patient-handling table that provides patient comfort and procedural utility; 4) a fully integrated Interventional System for MRI guided biopsy and localization; and 5) the user-friendly AuroraCAD™ computer-aided image display system designed to improve the accuracy and efficiency of diagnostic interpretations.'
Device Information and Specification
CONFIGURATION
Short bore compact
TE
From 5 ms for RODEO Plus to over 80 ms, 120 ms for T2 sequences
Around 0.02 sec for a 256x256 image, 12.4 sec for a 512 x 512 x 32 multislice set
20 - 36 cm, max. elliptical 36 x 44 cm
POWER REQUIREMENTS
150A/120V-208Y/3 Phase//60 Hz/5 Wire
| |  | |
• View the DATABASE results for 'Aurora® 1.5T Dedicated Breast MRI System' (2).
| | |
• View the NEWS results for 'Aurora® 1.5T Dedicated Breast MRI System' (3).
| | | | |  Further Reading: Further Reading: | News & More:
|
|
| |
|  | |  |  |  |
| |
|

From Hitachi Medical Systems America Inc.;
the AIRIS II, an entry in the diagnostic category of open MR systems, was designed by Hitachi
Medical Systems America Inc. (Twinsburg, OH, USA) and Hitachi Medical Corp. (Tokyo) and is manufactured by the Tokyo branch. A 0.3 T field-strength magnet and phased array coils de liver high image quality without the need for a tunnel-type high-field system, thereby significantly improving patient comfort not only for claustrophobic patients.
Device Information and Specification
CLINICAL APPLICATION
Whole body
QD Head, MA Head and Neck, QD C-Spine, MA or QD Shoulder, MA CTL Spine, QD Knee, Neck, QD TMJ, QD Breast, QD Flex Body (4 sizes), Small and Large Extrem., QD Wrist, MA Foot and Ankle (WIP), PVA (WIP)
SE, GE, GR, IR, FIR, STIR, FSE, ss-FSE, FLAIR, EPI -DWI, SE-EPI, ms - EPI, SSP, MTC, SARGE, RSSG, TRSG, MRCP, Angiography: CE, 2D/3D TOF
IMAGING MODES
Single, multislice, volume study
TR
SE: 30 - 10,000msec GE: 20 - 10,000msec IR: 50 - 16,700msec FSE: 200 - 16,7000msec
TE
SE : 10 - 250msec IR: 10 -250msec GE: 5 - 50 msec FSE: 15 - 2,000
0.05 sec/image (256 x 256)
2D: 2 - 100 mm; 3D: 0.5 - 5 mm
Level Range: -2,000 to +4,000
POWER REQUIREMENTS
208/220/240 V, single phase
COOLING SYSTEM TYPE
Air-cooled
2.0 m lateral, 2.5 m vert./long
| |  | |
• View the DATABASE results for 'AIRIS II™' (2).
| | | | |
|  | |  |  |
|  | 1 - 5 (of 22) nextResult Pages :  [1] [1]  [2 3 4 5] [2 3 4 5] |
| |
|
| |
 | Look
Ups |
| |